When we talk about ice bathing, there doesn’t necessarily have to be ice in the water. However, there must be a certain amount of cold to be able to speak of ice bathing or winter bathing, because a cold shower is not enough to put the body into the necessary state of shock and to unfold the positive effects and benefits of ice bathing.
Table of contents

Official ice bathing at a temperature of 5°C and below
Many people think of ice swimming as ice that has to be sawn or drilled beforehand in order to get into the water at all. This is not correct, as ice bathing or ice swimming starts at temperatures of 5 degrees Celsius and below. This guideline also applies to many associations, such as the ISA, which has written the 5 degrees Celsius into its association guidelines .
However, don’t be put off by the temperature, and not everyone has a thermometer with them to measure how cold the water is. If you take a cold shower at home in Germany, the water has an average temperature of around 15 degrees Celsius. This already feels cold to you, but ice bathing should be colder than your shower at home.
Below you will find a table with a few temperature limits that you can take into account:
| Water temperature | Description |
| 13 – 15°C | Cold shower Can be a little unpleasant at first, but you get used to the cold stimulus within about 30 seconds. Hypothermia is not possible. The easiest way to incorporate it into your daily routine is as an alternating shower and stop cold. |
| 10 – 12°C | Light ice bathing Pleasant temperature, depending on duration, no special knowledge required. Can also be achieved alone in the bath by adding a little ice from the freezer. |
| 6 – 9°C | Medium ice bath A good cold stimulus and a good feeling afterwards. Warming up after the medium ice bath is essential. Most ice bathers in Germany are probably in this cohort, as the lakes and rivers can easily fall into this temperature range in winter. |
| 3 – 5°C | Official ice swimming temperature Anything with a water temperature of 5°C and below qualifies for official participation in ice swimming events. The rule of thumb here is to stay in for the number of minutes that the water temperature is. Severe hypothermia can be the result of excessive ice swimming under these conditions. |
| 0 – 2°C | Siberian ice bathing Anything 2°C and below is painful and it is very easy to get hypothermia. At these temperatures, the water is typically a mix of ice and water, so you have to be careful not to cut yourself. Do not get your head and hair wet under any circumstances. |
If you are interested in how long ice bathing is good and healthy for you, you can read about it in our other article. The rule of thumb is: number of degrees Celsius = number of minutes in ice water.
How are density and volume related in water?
But let’s also take a closer look at the medium of water. It is a very unique element with many interesting characteristics. When we talk about density, we mean the molecules, in this case the water molecules, which are closer together at high density than at low density. When the molecules are closer together, the element is also the heaviest, as there is simply more mass in a smaller space. Volume refers to the expansion of the substance, i.e. how much space the substance takes up at what temperature. These two characteristics are particularly important if you want to better explain the behavior of water and go hand in hand.
Highest density and smallest volume at 4°C
Water is a very special element whose properties are not found in any other element. It has its highest density and smallest volume at 4 degrees Celsius. This means that the water molecules are closest together at this temperature and water is therefore the heaviest at 4°C. When water cools below 4°C, it expands again, contradictorily, and the density decreases again. The volume thus expands again below 4°C and the water becomes lighter again. This can be seen very clearly in the diagram below. Above 4°C, the density also decreases again and the volume increases.
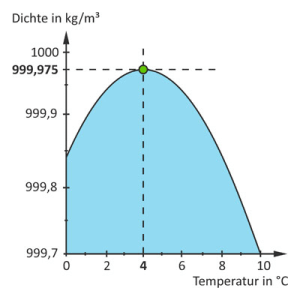
This is the peculiarity of water compared to all other substances, because normally the density (weight) of the molecules is higher the colder the substance is. So this is an anomaly to which we have become accustomed and which we sometimes lose sight of.
Consequently, ice, i.e. water at 0°C, has a lower density and is correspondingly lighter than water. As a result, huge icebergs can float on the surface of the water as they are lighter than the water in which the iceberg is located.

How does this manifest itself in your everyday life?
The phenomenon can be experienced in concrete terms if you put a closed bottle of beer or water in the freezer and risk it bursting because the volume of the liquid in the bottle increases when the liquid is cooled below 4°C.
The blasting effect of water can of course also be used positively, for example in arable farming for blasting and thus better aeration of the soil in winter.
Warm at the top in summer and cold at the bottom, the other way around in winter
To come back to ice bathing: When temperatures drop in winter, the water with the lowest density is pushed upwards (as it is lighter). So water with a temperature of less than 4 degrees Celsius rises, water with a temperature of more than 4 degrees Celsius sinks. It is therefore warmer at the bottom than at the top and the lake freezes from top to bottom.
This means that when ice swimming in the lake in winter, you have to bear in mind that the warmer water is at the bottom. It may therefore be that only a very thin layer is frozen at the top, which you could easily break through if you are careless.












Leave a Reply